Who are we and what do we do?
The National Research Ethics Committees (NREC) shall ensure that all research is conducted in accordance with recognized research ethical norms. We do preventive work, counselling, publish general and specific decisions and investigate individual cases concerning possible misconduct.
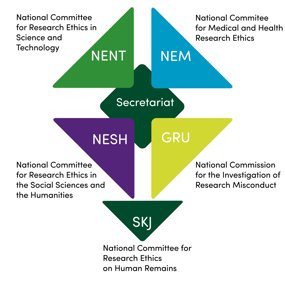
The National Research Ethics Committees are independent agencies for questions regarding research ethics, and investigation of misconduct, within all subject areas. The committees' shared secretariat is based in Oslo.
Committees and commission
We consist of:
National Committee for Medical and Health Research Ethics (NEM)
National Committee for Research Ethics in the Social Sciences and the Humanities (NESH)
National Committee for Research Ethics in Science and Technology (NENT)
National Commission for the Investigation of Research Misconduct
The committees also have an interdisciplinary committee that covers research on human remains:
National Committee for Research Ethics on Human Remains
In addition, there are seven independent regional committees for medical and health research ethics (REK), which give pre approval for research applications in these fields. NEM is the appeal body for REK.
Secretariat
The committees and the commission have a joint secretariat, based in Oslo.
Each committee has its own director with special expertise within the committee's field.
The secretariat also has expertise in administration, law and communication. It publishes the Research Ethics Magazine and has editorial responsibility for this website, including the Research Ethics Library (FBIB) - a resource for educators in research integrity and research ethics, students and others, with articles, discussion examples and more.
The website also contains research ethics guidelines, guides and other publications.
Coordinating function after the terrorist attacks 2011
National Research Ethics Committees was also given the task of coordinating research on the terrorist attacks July 22, 2011 where those directly affected participated. The primary objective was to safeguard the interests of those affected by the attacks in the research. This task was taken over by the Norwegian Directorate of Health in 2015 and ended in 2016.
Seeking advice
The committees and commission are advisory and anyone can seek advice.
When our committees are consulted in a specific case they will ask you to specify which research ethical aspects / challenges of the project you want them to consider first and foremost. If what one primarily wants is a general assessment, this should also be specified.
For research ethical issues in medicine and health sciences, contact NEM, NEM is also the complaints body for REK, which pre-approves medical and health sciences research projects.
For research ethical questions in the fields of science and technology, industrial, agricultural and fisheries research, as well as those parts of bio- and genetic engineering research that are not covered by medicine, contact NENT.
For research ethical issues in the social sciences and the humanities, including law and theology, contact NESH.
If you have any questions regarding suspected scientific misconduct, please contact the The National Commission for the Investigation of Research Misconduct. This commission is a supplement to local committees and an appeal body. The main responsibility lies with the research institutions.
When in doubt about the right addressee, contact post@forskningsetikk.no.
How the committees/commission work
The research ethics committees are broadly composed to reflect a diversity of views and values in society. They consist not only of researchers from different disciplines and institutions, but also of lay people's representatives. The members are appointed by virtue of their expertise, and represent none other than themselves.
In the committee work, national research ethics guidelines are a basic tool. The guidelines define the committees' scope and are explicitly used in justifying the assessments made. NEM and REK's work is based on the Helsinki Declaration, in addition to the Health Research Act. NEM has also developed area-specific guidelines and guides. NESH and NENT have developed subject-specific research ethics guidelines and additional area-specific guidelines and guides.
National Research Ethics Committees (NREC) is a governmental administrative body and has an obligation to comply with the Administration Act. This applies primarily to the handling of individual cases leading to administrative decisions. Even in counseling or statements in individual cases, general administrative law rules for case processing must be followed, among other things, a case must be well-informed and prepared, and parties must be given the opportunity to comment in the cases where they are entitled to.
NREC must also comply with the Freedom of Information Act. This states that "case documents, journals and similar registers of an administrative agency are public except as otherwise provided by statute or by regulations pursuant thereto. Any person may apply to an administrative agency for access to case documents, journals and similar registers of that administrative agency". Our public records are published online every week.